
You've reached the Virginia Cooperative Extension Newsletter Archive. These files cover more than ten years of newsletters posted on our old website (through April/May 2009), and are provided for historical purposes only. As such, they may contain out-of-date references and broken links.
To see our latest newsletters and current information, visit our website at http://www.ext.vt.edu/news/.
Newsletter Archive index: http://sites.ext.vt.edu/newsletter-archive/

Effects of Type of Sow Gestation Housing on Growth Performance and Reproduction in Gilt Offspring
Livestock Update, September 2008
Mark J. Estienne, Ph.D. and Allen F. Harper, Ph.D., VA Tech Tidewater AREC
![]()
Introduction
An exciting and growing body of scientific evidence supports the notion that the maternal environment in which fetuses develop plays a profound role in the development of the reproductive and other physiologic systems. Fetal programming refers to the process by which an acute or chronic stimulus in utero (i.e., in the uterus) establishes a permanent response in the fetus that impacts physiologic function later in life. The concept of fetal programming in swine is illustrated by an experiment reported by O’Gorman et al. (2007). In that investigation, gestating crossbred sows were allocated to one of two treatment groups: control or “stressed”. Stressed sows were subjected to daily restraint for five minutes during weeks 12 to 16 of gestation. Average age at first estrus was significantly delayed in gilts farrowed by stressed sows (~172 days) compared to gilts farrowed by control females (~158 days).
For an experiment conducted at TAREC, Estienne et al. (2006) reported that pregnancy rate was higher for gilts housed in individual stalls (100%) compared with group-housed individuals (85.7%). However, serum concentrations of cortisol, a classical “stress” hormone tended to be greater in gilts housed in stalls (79.4 versus 57.1 ng/mL). A working hypothesis in our laboratory is that if there is indeed a difference between housing systems (individual stalls versus group pens) in terms of “stress” to, and well-being of, the sow, than due to fetal programming, growth and reproductive performance of offspring may be impacted.
Thus, the objective of this experiment was to determine the effects of type of sow gestation housing on various characteristics of female offspring including growth performance and the age at puberty.
Materials and Methods
Yorkshire x Landrace gilts were mated by artificial insemination (AI) and allotted to one of three types of gestation housing: I. individual stalls throughout gestation, II. group pens throughout gestation (5 to 6 gilts/pen); or III. individual stalls for 30 days post-mating and then group pens for the remainder of gestation. At day 110 of gestation, gilts were moved to the farrowing barn. Barrow pigs were cross-fostered among litters within a treatment group so that sows were nursing an approximately equal number of pigs (10.5 + 0.3; mean + SE).
At weaning (24.6 ± 0.3 days of age), gilts were placed in nursery pens each containing three pigs farrowed exclusively by gilts exposed to one of the three gestation housing systems described above. Average daily gain, feed consumption and feed conversion efficiency were determined. Following the five-week nursery trial, intact pens of pigs were moved to a grower-finishing barn. The grow-finish period ended when gilts weighed an average of ~240 lb, at which time backfat thickness was determined using A-mode ultrasonography (Sono-grader, Renco Inc., Minneapolis, MN); Average daily gain, feed consumption and feed conversion efficiency were determined.
After the grow-finish trial, gilts were checked for estrus once daily in the presence of a boar and age at puberty (first standing estrus in the presence of the boar) determined. Ten days after estrus, gilts were killed and reproductive tracts collected and evaluated as previously described (Estienne et al., 2001). All data were analyzed using SAS (SAS Institute, Inc., Cary, NC).
Results
Farrowing data for the experimental females is contained in Table 1. There were no effects of housing on litter size, although numerically, there were a greater number of pigs born alive for females kept in stalls throughout gestation or in stalls for the first thirty days post-mating and group pens for the remainder of pregnancy, compared to gilts kept in group pens throughout gestation. Among treatments, the body weights of gilts pigs were similar at birth and at weaning.
During the nursery phase of production, there was no effect of treatment on growth rate, feed consumption or feed conversion efficiency (Table 2).
Growth performance for the grow-finish period is summarized in Table 3. Overall, average daily gain was not affected (P = 0.13) by treatment. However, the pattern of pig growth was affected by gestation housing of dams as evidenced by a housing type by time interaction (P = 0.04), with pigs farrowed by gilts housed in stalls throughout gestation weighing more during the last two weeks of the grow-finish period (Figure 1).
There was no effect of treatment for feed consumed, however, feed conversion efficiency was enhanced (P < 0.05) in gilts farrowed by females kept in stalls throughout gestation or in stalls for the first thirty days of gestation and pens for the remainder of pregnancy, compared to gilts farrowed by females kept in group pens throughout gestation. Pigs farrowed by females kept in stalls throughout gestation tended (P < 0.09) to have less backfat than gilts farrowed by females kept in group pens throughout gestation, with gilts from females kept in stalls for the first thirty days of gestation and pens for the remainder of pregnancy having an intermediate value that was not different from the other two groups.
Reproductive traits for gilts farrowed by sows housed during gestation in the various systems are contained in Table 4. The average weight of ovaries (P = 0.07) and the weight of follicular fluid (an indication of follicle number and/or size) (P = 0.08) tended to be greater for gilts farrowed by sows housed in groups throughout gestation.
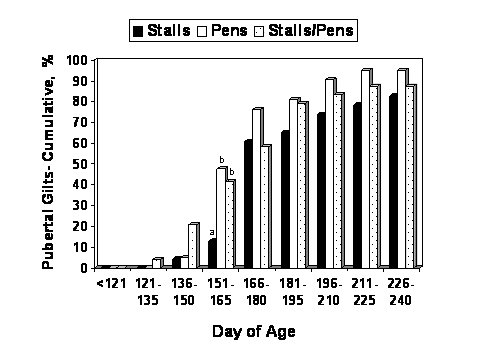
The average age at puberty did not differ among groups (P = 0.61; Table 4), however, fewer gilts farrowed by females that were gestated in stalls reached puberty by 165 days of age compared with the other two groups. Figures 2 and 3 depicts for the different treatment groups, the percentage of gilts reaching puberty at various age ranges, and the cumulative percentage of gilts reaching puberty by various age ranges, respectively.
The number of corpora lutea (an indication of ovulation rate) (P = 0.55), average size of corpora lutea (P = 0.41), and uterine weight (P = 0.42), were similar among groups.
Discussion
Individually housing pregnant females result in certain production advantages, and it is estimated that at least 60% of the sows and gilts in the U.S. are kept in stalls throughout gestation (Barnett et al., 2001). This method of sow housing, however, is the most contentious welfare issue facing pork producers. Typical gestation stalls measure 2’ x 7’ and limit sows to standing, sitting, and lying. This restricted freedom of movement has been, and continues to be, robustly criticized by animal rights and animal welfare activists who proclaim that gestation stalls are inherently stressful and do not provide for sow well-being. On the basis of a comprehensive review of the scientific literature, however, McGlone et al. (2004) concluded that well-managed stalls or group pens generally produce similar states of well-being for pregnant sows in terms of physiology, behavior, performance, and health. However, the effects of housing type on the performance of offspring have not been previously reported.
Indeed, fetal programming is the process by which a stimulus in utero establishes a permanent response in the fetus impacting physiology later in life. Results of the current project illustrate that the type of housing to which pregnant gilts are exposed affects the performance of gilt offspring. Although growth performance during the lactation and nursery phases of production did not appear to be impacted by housing type, growth rates during the late finishing phase were greater for gilts farrowed by sows gestated in stalls compared with the other groups. Moreover, gilts farrowed by females kept in stalls throughout pregnancy were leaner at market weight. These findings are consistent with the concept that gestation housing does indeed affect gilt offspring performance via fetal programming, and the effects are manifested later in life and not during early postnatal growth. Consistent with this hypothesis, in a review of literature, Foxcroft and Town (2004) concluded that variation in growth performance after birth is pre-programmed during fetal development in the uterus and it is likely that these pre-programmed limitations in growth performance express themselves in the late grower or finisher stages of production.
In contrast, gilts farrowed by females that gestated in group pens had larger ovaries, greater follicular development, and reached puberty sooner than did gilts housed in stalls. In general, reproductive characteristics of gilts that were farrowed by females that were gestated in stalls for the first thirty days post-mating and then group pens for the remainder of pregnancy were intermediate between the two other groups.
In summary, the type of housing to which pregnant gilts are exposed affects subsequent growth and reproduction in female offspring perhaps through a phenomenon referred to as fetal programming. In general, gilts farrowed by females kept in stalls during gestation grew faster and were leaner at market weight, but displayed delayed puberty. The exact physiologic mechanisms responsible for these effects remain to be elucidated. The results reported here offer insight into the emerging concept of fetal pig programming and findings could impact future housing systems, particularly for those producers contemplating a switch from individual to group gestation housing.
Literature Cited
Barnett, J.L., P.H. Hemsworth, G.M. Cronin, E.C. Jongman, and G.D. Hutson. 2001. A review of the welfare issues for sows and piglets in relation to housing. Australian Journal of Agricultural Research 52:1-
28.
Estienne, M.J., and A.F. Harper. 2006. Reproductive traits in gilts housed individually or in groups during the first thirty days of gestation. Journal of Swine Health and Production 14:241-246.
Estienne, M.J., A.F. Harper, B.R. Horsley, C.E. Estienne, and J.W. Knight. 2001. Effects of P.G. 600 on the onset of estrus and ovulation rate in gilts treated with regu-mate. Journal of Animal Science79:2757-2761.
Foxcroft, G.R., and S.C. Town. 2004. Prenatal programming of postnatal performance- The unseen cause of variance. Advances in Pork Production 15:269-279.
McGlone, J.J., E.H. von Borell, J. Deen, A.K. Johnson, D.G. Levis, M. Meunier-Salaun, J. Morrow, D. Reeves, J.L. Salk-Johnson, and P.L. Sundberg. 2004. Review: Compilation of the scientific literature comparing housing systems for gestating sows and gilts using measures of physiology, behavior, performance, and health. Professional Animal Scientist 20:105-117.
O’Gorman, C.W., E. Gonzales, M.D. Eaton, K.A. Collard, M. Reyna, J.C. Laurenz, R.L. Stanko, D.H. Keisler, J.A. Carroll, and M.R. Garcia. 2007. Fetal exposure to maternal stress influences leptin receptor gene expression during development and age at puberty in gilts. Journal of Animal Science 85(Suppl. 2):13.
Table 1. Farrowing data for females that were kept in individual stalls or group pens throughout gestation or that were kept in individual stalls for the first 30 days after mating and then group pens for the remainder of gestation.
Item: |
Stalls |
Pens |
Stalls/Pens |
SE |
P |
Number |
6 |
6 |
7 |
--- |
--- |
Litter Size |
|
|
|
|
|
Total |
12.8 |
11.7 |
12.9 |
1.0 |
0.62 |
Born Alive |
11.8a |
9.2b |
11.4a |
0.9 |
0.11 |
Still Born |
0.8 |
1.7 |
0.6 |
0.6 |
0.37 |
Mummified fetuses |
0.2 |
0.8 |
0.9 |
0.4 |
0.32 |
Weaned |
9.5 |
9.8 |
9.7 |
0.5 |
0.89 |
Weight of gilt pigs, lb |
|
|
|
|
|
Birth |
3.6 |
3.5 |
3.8 |
0.2 |
0.46 |
Weaning |
19.3 |
19.3 |
18.7 |
1.0 |
0.86 |
Table 2. Nursery performance for gilts farrowed by females that were kept in individual stalls or group pens throughout gestation, or individual stalls for the first 30 days after mating and then group pens for the remainder of gestation.
Item: |
Stalls |
Pens |
Stalls/Pens |
SE |
P |
Number of pens1 |
9 |
9 |
9 |
--- |
--- |
Daily gain, lb |
1.16 |
1.15 |
1.15 |
0.03 |
0.93 |
Daily feed consumed, lb |
2.01 |
2.02 |
2.00 |
0.06 |
0.95 |
Feed/gain |
1.73 |
1.75 |
1.75 |
0.03 |
0.86 |
1Each pen contained three gilts each.
Table 3. Grow-finish performance for gilts farrowed by females that were kept in individual stalls or group pens throughout gestation, or individual stalls for the first 30 days after mating and then group pens for the remainder of gestation.
Item: |
Stalls |
Pens |
Stalls/Pens |
SE |
P |
Number of pens1 |
9 |
9 |
9 |
--- |
--- |
Daily gain, lb |
2.21 |
2.11 |
2.11 |
0.04 |
0.13 |
Daily feed consumed, lb |
5.75 |
5.76 |
5.55 |
0.14 |
0.54 |
Feed/gain |
2.60a |
2.73b |
2.62a |
0.04 |
0.05 |
Backfat thickness2, mm |
10.9c |
12.5d |
12.1c,d |
0.5 |
0.09 |
1Each pen contained three gilts each.
2Measured ultrasonically at the last rib at the end of the grow-finish trial.
a,bMeans with different superscripts differ (P < 0.05).
c,dMeans with different superscripts tend to differ (P < 0.09).
Table 4. Reproductive characteristics for gilts farrowed by females that were kept in individual stalls or group pens throughout gestation, or individual stalls for the first 30 days after mating and then group pens for the remainder of gestation.
Item: |
Stalls |
Pens |
Stalls/Pens |
SE |
P |
Number of pens1 |
9 |
9 |
9 |
--- |
--- |
Avg. weight of ovaries, g |
9.1a |
10.8b |
9.9a,b |
1.0 |
0.07 |
Corpora lutea |
15.3 |
16.1 |
14.9 |
0.7 |
0.55 |
Avg. weight of corpora lutea, g |
0.57 |
0.46 |
0.51 |
0.06 |
0.41 |
Follicular fluid weight, g |
2.66c |
4.55d |
3.05c |
0.59 |
0.08 |
Weight of uterus, g |
583.9 |
530.6 |
557.0 |
28.0 |
0.42 |
Age at puberty, days |
175.4 |
170.7 |
169.6 |
4.4 |
0.61 |
Number of gilts not pubertal by 240 days of age |
4 |
1 |
3 |
--- |
--- |
1Each pen contained three gilts each.
a,bMeans with different superscripts tend to differ (P < 0.07).
c,dMeans with different superscripts tend to differ (P < 0.08).
Figure 1. Body weights during the grow-finish period for gilts farrowed by females that were kept in individual stalls or group pens throughout gestation, or individual stalls for the first 30 days after mating and then group pens for the remainder of gestation. Values are means (n = 9 pens/group; SE = 1.48). A significant housing type by time interaction was detected (P = 0.04). Means marked by an *, indicate body weights for gilts farrowed by females kept in stalls throughout pregnancy were greater (P < 0.05) than the weights for gilts in the other two groups
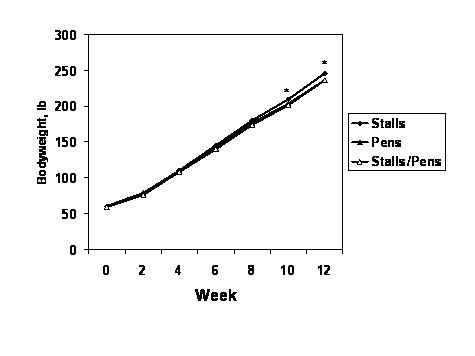 |
Figure 2. Percentage of gilts attaining puberty at various ages for gilts farrowed by females that were kept in individual stalls or group pens throughout gestation, or individual stalls for the first 30 days after mating and then group pens for the remainder of gestation. For the period between 151 and 165 days of age more (P < 0.05) gilts farrowed by females gestated in pens reached puberty than did gilts farrowed by females housed in stalls throughout gestation, with gilts farrowed by females housed in stalls for the first thirty days of gestation and pens for the remainder of pregnancy having an intermediate value that was not different from the other two groups. Within an age range, means marked by different superscripts (a, b) differ (P < 0.05).
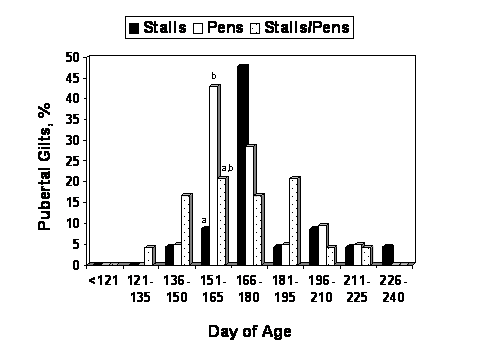 |
Figure 3. Cumulative percentage of gilts attaining puberty by various ages for gilts farrowed by females that were kept in individual stalls or group pens throughout gestation, or individual stalls for the first 30 days after mating and then group pens for the remainder of gestation. For the period between 151 and 165 days of age fewer (P < 0.05) gilts farrowed by females gestated in stalls throughout gestation had reached puberty compared to the other two groups. Within an age range means marked by different superscripts (a, b) differ(P < 0.05).
 |